Abstract
Background CD19 CAR T-cells can induce prolonged remissions and potentially cure a significant proportion of patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCL). However, some patients may die of causes unrelated to lymphoma after CAR T-cell therapy. To date, little is known about the non-relapse mortality (NRM) following CAR T-cells. Improving our knowledge of NRM may help guide the selection and management of patients in order to improve their outcome after CAR T-cell therapy. Using the French DESCAR-T registry, we analyzed the incidence and causes of NRM, and identified risk factors of NRM.
Methods We retrospectively analyzed patients from the DESCAR-T registry who received commercial CD19 CAR T-cells for DLBCL between July 2018 and April 2022, from 27 French centers. NRM was defined as patients who died of causes unrelated to lymphoma relapse/progression (deaths of unknown origin were excluded) within 28 days from CAR T-cell infusion (early NRM) or beyond (late NRM). Overall NRM was defined as the sum of early and late NRM. Descriptive statistics of early and overall NRM were performed and cumulative incidences calculated. Risk factors for early and overall NRM were identified by comparing the characteristics at lymphodepletion of early and overall NRM population with patients alive at day 28 post-CAR T-cell infusion or alive at 1 year following CAR-T cell infusion, respectively.
Results Between July 2018 and April 2022, 977 consecutive patients registered in the DESCAR-T registry received commercial CD19 CAR T-cells for DLBCL, either axicabtagene ciloleucel (axi-cel, N=611) or tisagenlecleucel (tisa-cel, N=366). Median age was 62 years (range, 18-82), 600 (61%) patients were male, 94 (10%) had an Eastern Cooperative Oncology Group performance status (ECOG PS) >1, 55 (5.6%) had an International Prognostic Index (IPI) >3, 459 (47%) had received ≥3 prior lines of treatment, 118 (12%) had failed a prior autologous stem cell transplant.
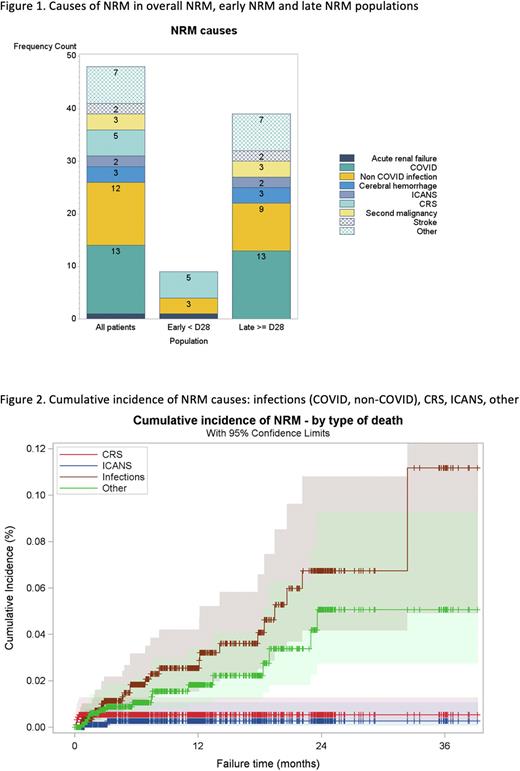
After a median follow-up of 12.4 months, 48 (4.9%) patients had died of causes unrelated to lymphoma and defined the overall NRM population: 9 deaths (0.92% of all patients and 19% of all NRM) occurred before day 28 post-infusion (early NRM population) and 39 deaths (3.99% of all patients and 81% of all NRM) occurred beyond day 28 post-infusion (late NRM population). Causes of early NRM were cytokine release syndrome (CRS) in 5 patients, infections in 3 patients (2 bacterial sepsis and 1 bacterial pneumonia), and acute kidney failure in one patient. Risk factors for early NRM were as follows: older age (median 73 years vs 63 years, p=0.020), lower body mass index (median 22.5 vs 24.4, p=0.040), ECOG PS >1 (56% vs 13%, p=0.003), higher aspartate transaminase levels (median 68 vs 22 IU/L, p=0.012), higher total bilirubin levels (median 10 vs 6 µmol/L, p=0.035), higher LDH levels (median 408 vs 250 IU/L, p=0.019), and higher ferritin levels (median 1837 vs 480 µg/L, p=0.021).
Causes of overall NRM were COVID infection in 13 patients, non-COVID infection in 12 patients (6 bacterial sepsis, 4 bacterial pneumonia, one peritonitis, one septic arthritis), CRS in 5 patients, second malignancy in 3 patients, cerebral hemorrhage in 3 patients, stroke in 2 patients, immune effector cell associated neurotoxicity (ICANS) in 2 patients, acute kidney failure in 1 patient and deaths from other causes in 7 patients. Risk factors for overall NRM were as follows: older age (median 67 years vs 63 years, p=0.003), diabetes (23% vs 10%, p=0.025), ECOG PS >1 (23% vs 5%, p<0.001), lower platelet counts (median 156 g/L vs 185 g/L, p=0.034), higher C-reactive protein levels (median 17 vs 5 mg/L, p<0.001), higher ferritin levels (median 732 vs 393 µg/L, p=0.002), and higher gamma-glutamyl transferase (GGT) levels (median 50 vs 30 IU/L, p=0.017).
Conclusion To our knowledge, this is the largest study focusing on NRM after CAR T-cell therapy. NRM in patients receiving commercial CAR T-cells for DLBCL is a relatively rare event (4.9%). Most NRM occurs late (81%) and is due to infections (52%). We identified risk factors of early and overall NRM, which can be useful to select patients for CAR T-cell therapy and prevent/reduce the risk of NRM and thus ultimately improve the outcome of patients treated with CAR T-cells.
Disclosures
Bachy:Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen, BMS: Research Funding; Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Hospices Civils de Lyon: Current Employment. Cartron:Gilead, Novartis, Mylteni, Sanofi, Abbvie, Takeda, Roche, Janssen, Celgene, Novartis, Bristol Myers Squibb: Honoraria; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Beauvais:Gilead: Honoraria. Gastinne:Gilead/Kite, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings/travel, participation in a data safety monitoring board or advisory board. Leleu:Sanofi: Honoraria; Janssen: Honoraria; Takeda: Honoraria; BMS: Honoraria; Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria. Mohty:Takeda: Honoraria; Novartis: Honoraria; Astellas: Honoraria; Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Amgen: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Gilead: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Casasnovas:Roche, Gilead, Takeda: Research Funding; Roche, Takeda, BMS, MSD, Gilead/Kite, Janssen, ADC Therapeutics, Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche, Takeda, Merck, BMS, Gilead/Kite, Abbvie, ADC therapeutics, INCYTE, AstraZeneca: Honoraria. Castilla-LLorente:IXAKA LIMITED: Consultancy; GILEAD KITE: Honoraria. Hermine:BMS: Honoraria, Research Funding; Novartis: Research Funding; Kite/Gilead: Honoraria; Inatherys: Research Funding; AB Science: Current equity holder in private company, Honoraria, Research Funding. Bories:Novartis, Kite/Gilead, BMS-Celgene, Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Herbaux:Takeda: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; MSD: Research Funding; Roche: Honoraria; Janssen: Honoraria; Gilead: Honoraria; Kite: Honoraria. Sesques:Chugai, Novartis, and Kite/Gilead: Research Funding. Le Gouill:Novartis, Kite/Gilead, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Morschhauser:Roche, Chugai: Other: Scientific lectures; gilead, roche: Consultancy; Gilead, Novartis, BMS, épizyme, miltenyi, Abbvie, genmab, Roche, AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Thieblemont:Amgen: Consultancy, Honoraria; Incyte: Honoraria; Bayer: Honoraria; Kite: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Honoraria. Houot:Janssen: Honoraria; Novartis: Honoraria; Kite: Honoraria; Gilead: Honoraria; MSD: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.